Expansion microscopy
Instead of focusing in increasing the resolution of optical microscopes, one could simply make the sample bigger. With this approach it becomes simple to overcome the diffraction limit even in inexpensive microscopes.
To expand the sample, it is possible to use a hydrogel (see: Hydrogels, hydrogels can encapsulate live cells) that gets swollen after it has permeated the cell membrane. The trick is to use a cross linker that fixes that mesh before water is encapsulated[@wassie2019Expansion microscopy: principles and uses in biological research].
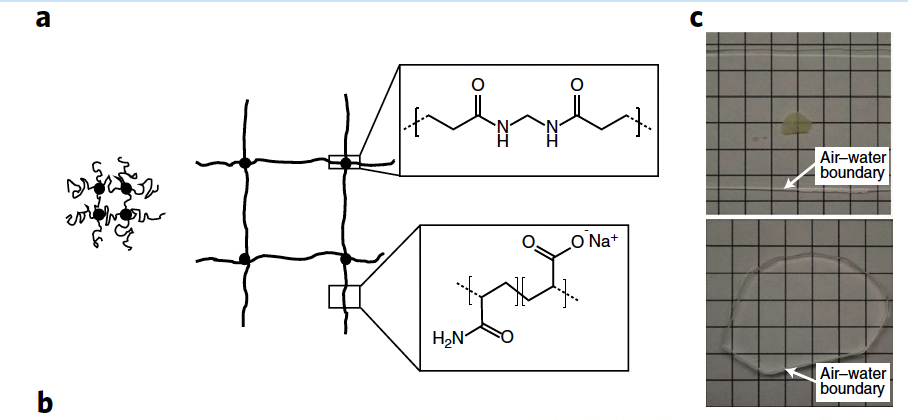
The volume the samples grow is of around 100X, which means a linear expansion of 4.5X. In a diffraction limited microscope with 300nm resolution, this translates to 70nm resolution. If the process is applied twice, the resulting resolution is of just 15nm.
In order for the process to work, the labels must be anchored to the hydrogel such that during expansion, the relative distances are preserved. This somewhat limits the number of dyes that can be used. Still, protocols are being released all the times to include new labels. A side-effect of the process is that cells become transparent, making them ideal candidates for light sheet microscopes.
Moreover, it is possible to combine super-resolution techniques with the expansion method, achieving a real-space resolution of few nanometers.
On the down side, this method only works with dead (fixed) cells, therefore not suitable for live imaging, etc. On the other hand, the method can be used to de-crowd protein environments, giving access to novel insight. It can also be coupled to FISH microscopy, used for pathology, etc.
The overall precision of the method can be of as much as 5nm to 10nm. Mostly limited by how isotropic the expansion process is[@wassie2019Expansion microscopy: principles and uses in biological research].
See more: https://www.expansionmicroscopy.org/
Backlinks
These are the other notes that link to this one.
