Pem water electrolyzers
There is an overarching idea of using hydrogen as an intermediate product in many chemical processes ([[@vogt2024The refinery of the future]])
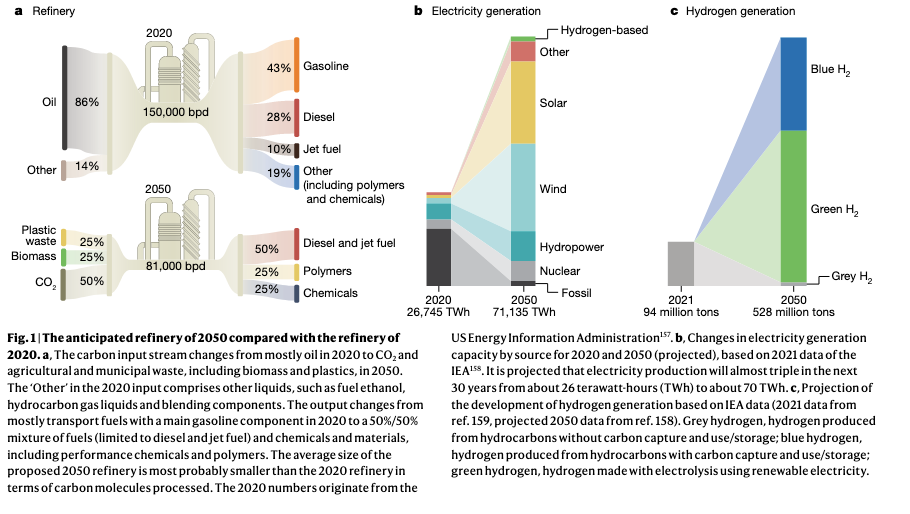 Therefore, hydrogen is an intermediate step that would enable the creation of crucial products. One of the challenges is that the creation of hydrogen requires both electric power, and expensive catalysts. Both topics prevent it from scaling compared to the use of fossil fuels.
Therefore, hydrogen is an intermediate step that would enable the creation of crucial products. One of the challenges is that the creation of hydrogen requires both electric power, and expensive catalysts. Both topics prevent it from scaling compared to the use of fossil fuels.
PEM Water Electrolysis has the promise of being intermitten-friendly, which means it can be hooked into renewable sources of electricity, using the surplus when available. However, power demand to run a PEM plant is non negligible, which means that during transition periods (when intermittent power is not available at industrial scale) there is no economic path towards success.
The other challenge is the reliance on #iridium , an extremely scarce metal that is mined in South Africa and currently yields around 7 tons per year. To unlock the power of Iridium-based PEM electrolysers, either new sources of iridium are found, alternatives are discovered, or processes that reduce the amount required are introduced.
VSParticle is working towards lowering the iridium requirements for PEM. The idea is that a nanoporous layer can approach the optimum utilization, by exposing the maximum amount of surface area to water and allowing the percolation of the gas from one side to the other of the membrane.
Others, such as Lila Sciences are working on developing materials with the same properties but avoiding iridium altogether.
Hydrogen can also be used in fuel cells as a replacement of both batteries and fossil fuels (see: 10 actions regarding climate change and: 202111292230 diesel is the most efficient energy storage solution for transport).
The question that remains is whether generating hydrogen from green electricity will be economically viable in the near future.
