Literature/202405110829 cytometer to measure full empty ratio of lnp
- Source: [[@li2022aPayload distribution and capacity of mRNA lipid nanoparticles]]
- Tags: #lipid-nano-particle #full-empty-ratio
This paper implements a flow cytometer inside a capillary. I am not completely sure about the advantages of their approach compared to any other tool, such as nanoFCM.
The capillary is a Molex glass capillary with a
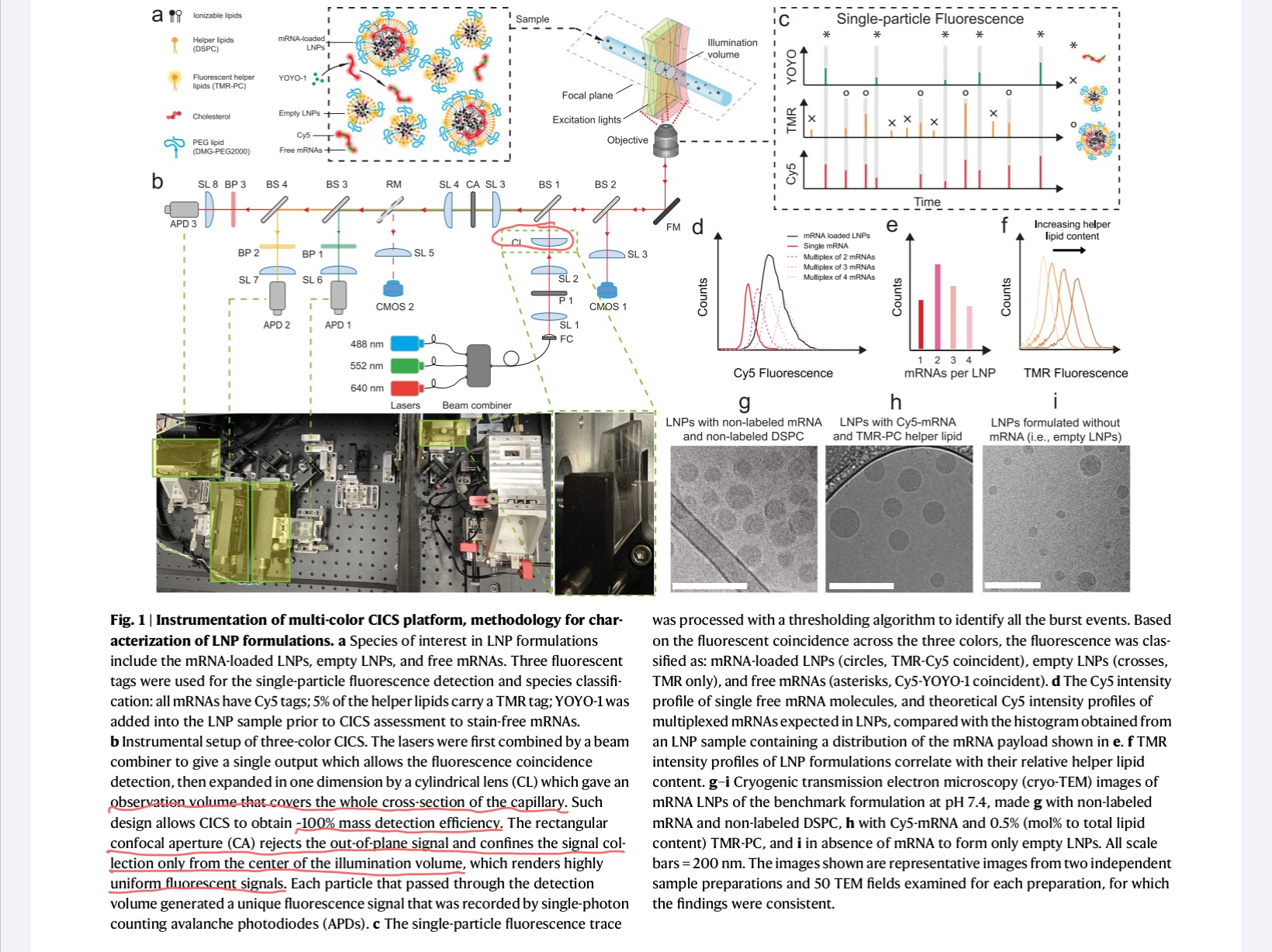 There is some discussion about aligning the capillary to the detectors, but I am still missing a discussion on how they compensate the planar geometry while using point detectors. Even they claim using a rectangular aperture to clean up the background, but there is no secondary cylindrical lens.
There is some discussion about aligning the capillary to the detectors, but I am still missing a discussion on how they compensate the planar geometry while using point detectors. Even they claim using a rectangular aperture to clean up the background, but there is no secondary cylindrical lens.
The rest of the paper is mostly a discussion on specific samples and the distribution of mRNA on each lipid nano particle. The equation they derive is the following:
$$
D_{LNP} = \sum^{N}{n=1}\omega{n}\times D_{RNA, n}
$$
Where the distribution of number of RNA molecules per particle is computed by calculating the weights that must be assigned to the signal of different numbers of RNA in the focal volume. They also claim that
I think all this discussion is rather naïve, since the only thing it gets is that the way of measuring the number of RNA molecules in a particle is a direct multiplication by the intensity given by a single RNA molecule.
There's just a slight complication since intensities themselves forma distribution and not a single value, but the fact they don't have an experimental basis (they neglect any effects in having dual fluorophores, self-quenching, etc.)
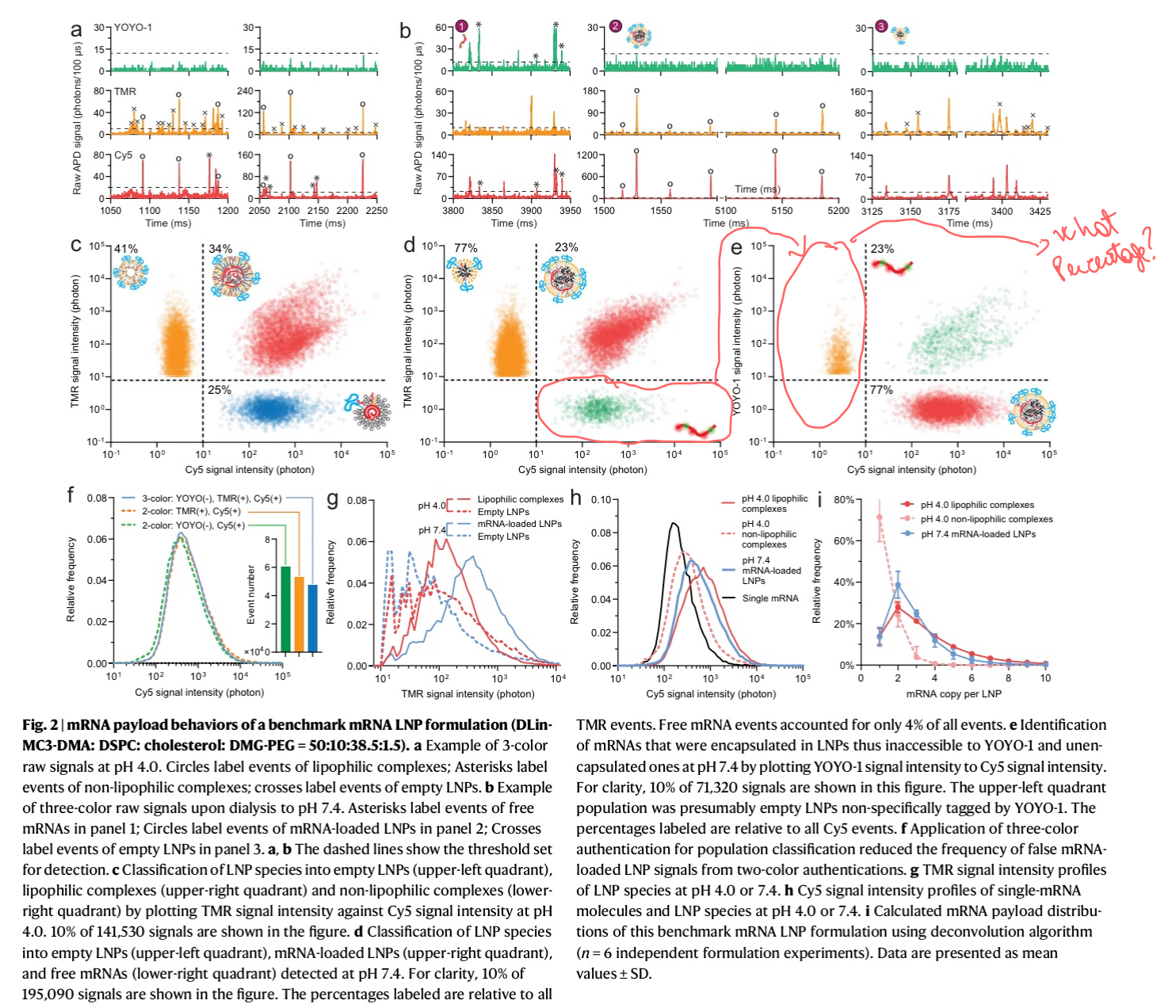
The data in the paper looks exactly like it would look like in a flow-cytometer experiment, but without scattering (using scattering instead of fluorescence).
The important conclusion of the paper is that 80% of the LNP's are empty, even if they were using "the gold standard" of mRNA in LNP's.
Tech Specs (at least some)
- Throughput: 3000~5000 events/minute
- Excitation:
- Molex Capillary (Polymicro, I assume),
- No mention to index-matching the capillary to a flat substrate (but they mention a coverslip supporting it)
Note
The paper goes as far as describing a fitting procedure that minimizes
